| |
 |
| Issue 15 |
|
June 2000
|
| |
 |
| Recent studies support scientific basis of the CRASH Trial |
 |
Two recent studies, reported in the
BMJ, strongly support the scientific basis of the CRASH trial. The
first, a review of head injury trials by Dickinson et al, found that existing
trials are too small to reliably detect moderate treatment effects. The
average number of patients per trial was 82, and the largest trial conducted
to date included only about 1000 patients. No trials were large
enough to detect reliably a 5% absolute reduction in the risk of death
or disability. If successful, CRASH will be the first head injury trial
large enough to detect moderate but clinically important treatment effects.
|
| The second study by Thornhill
et al identified a cohort of young people and adults admitted to hospital
with head injury and followed up groups representative of initially mild,
moderate, and severe head injuries to determine outcome one year later.
They found a
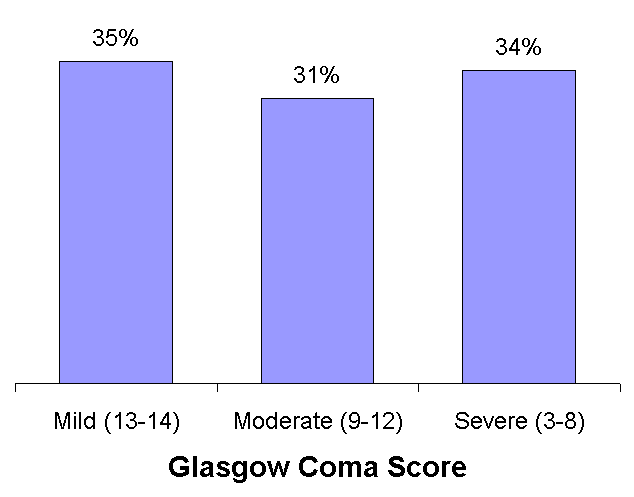
high frequency of disability after mild as well as moderate and severe injuries. The authors point out that classing a head injury as "mild" when the Glasgow coma score (GCS) is 13-15 on admission to hospital is inappropriate in many instances. The high frequency of disability after "mild" head injury is a strong argument for the broad inclusion criteria in the CRASH trial. Patients are eligible for inclusion in CRASH if they are observed in hospital to have a GCS of 14 or less. The trial therefore includes patients with mild, moderate and severe head injury. The graph shows the distribution of GCS at trial entry in the CRASH trial and shows an even distribution of patients with mild, moderate and severe head injury. Assessing the effect of corticosteroids on disability is an important aim of the CRASH trial and the paper by Thornhill underscores the importance of doing so. Dickinson K, Bunn F, Wentz R,
et al. Size and quality of randomised controlled trials in head injury:
review of published studies. BMJ 2000; 320: 1308-1311
|
| RECRUITMENT NEWS
The landmark 500th patient was randomised by Trafford General Hospital - Congratulations! Dr María de los Angeles Muñoz-Sánchez in Trauma Centre ‘Virgen del Rocio’ in Spain randomised their first patient this month. Congratulations to Pavlos Myrianthefs in KAT Hospital of Athens and Jafri Malin Abdullah in Hospital Universiti Sains Malaysia. Both have received Ethics Committee Approval recently and will start randomising shortly. Congratulations, too, to St
James’s University Hospital in Dublin, who are in the top five of the UK
recruitment league, and yet, have managed to keep 100% up to date with
Early Outcome Forms and data queries.
|
QUESTIONS COLUMN
Question: What do we do if a relative objects to a patient being enrolled in the trial? Answer: If a relative is present when a
patient is being considered for trial entry, and the relative objects to
the patient being in the trial, the patient should not be entered. If a
relative arrives in hospital after a patient has been enrolled, and objects
to the patient being in the trial, an urgent meeting is arranged with a
senior clinician to discuss the trial, and if the relative still objects,
the trial treatment should be stopped.
|
|
CRASH Co-ordinating Centre, FREEPOST LON 14211, LONDON WC1N 1BR Tel: + 44 (0)20 7299 4684 Fax: + 44 (0)20 7299 4663 email: CRASH@lshtm.ac.uk |