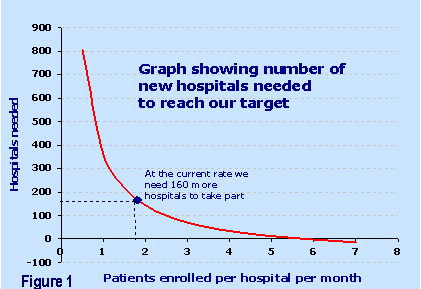
-
Research
Institute for Special Surgery and Trauma (Czech Republic) 292
-
Centre
Hospitalier Regional de Namur (Belgium) 192
-
Hope
Hospital (UK) 117
-
Tygerberg
Academic Hospital (South Africa) 92
-
Birmingham
Heartlands Hospital (UK) 75
-
University
Hospital of Zurich (Switzerland) 65
-
North
Manchester General Hospital (UK) 64
-
St
James's University Hospital (Eire) 57
-
Wentworth
Hospital (South Africa) 57
-
Whiston
Hospital (UK) 50
-
Batticaloa
General Hospital, Teaching (Sri Lanka) 48
-
Trauma
Centre - Virgen Del Rocio (Spain) 48
-
Clinica
Las Americas (Colombia) 43
-
Hospital
University Science Malaysia (Malaysia) 39
-
National
Hospital of Sri Lanka (Sri Lanka) 39
-
Spitalul
Clinic de Urgenta Bucuresti (Romania) 38
-
Colchester
General Hospital (UK) 35
-
Reimann
Hospital (Slovakia) 33
-
Eastbourne
District General Hospital (UK) 32
-
Royal
Bolton Hospital (UK) 31
-
Mataria
Teaching Hospital (Egypt) 30
-
Selly
Oak Hospital (UK) 30
-
Suez
Canal University (Egypt) 28
-
Royal
Albert Edward Infirmary (UK) 26
-
Stepping
Hill Hospital (UK) 24
-
Trafford
General Hospital (UK) 23
-
Hospital
General de Medellin (Colombia) 18
-
Masaryk
Hospital (Czech Republic) 17
-
Blackburn
Royal Infirmary (UK) 16
-
Royal
Oldham Hospital (UK) 16
-
Hospital
San Bernardo (Argentina) 16
-
A.Z.
Kliena Hospital (Belgium) 15
-
Countess
of Chester Hospital (UK) 14
-
Cheltenham
General Hospital (UK) 13
-
Queen
Elizabeth The Queen Mother
-
Hospital
(UK) 13
-
Bury
General Hospital (UK) 13
-
Northern
General Hospital (UK) 13
-
Princess
Alexandra Hospital (UK) 13
-
Furness
General Hospital (UK) 12
-
Queen
Elizabeth Hospital, Birmingham (UK) 11
-
Lagos
University Teaching Hospital (Nigeria) 11
-
Worthing
Hospital (UK) 10
-
Royal
Liverpool University Hospital (UK) 9
-
Hospital
Escuela (Argentina) 8
-
Ninewells
Hospital and Medical School (UK) 8
-
Aberdeen
Royal Infirmary (UK) 8
-
Hospital
Pribram (Czech Republic) 7
-
Ernst
Moritz Arndt University (Germany) 7
-
Hospital
Municipal "Dr Leonidas Lucero" (Argentina) 7
-
University
Hospital Hradec Kralove (Czech Republic) 7
-
Hospital
Italiano (Argentina) 6
-
National
Neuroscience Institute (Singapore) 6
-
Hospital
of Jolimont (Belgium) 6
-
Medway
Maritime Hospital (UK) 5
-
Dunedin
Hospital (New Zealand) 5
-
Withybush
General Hospital (UK) 4
-
Hospital
Municipal Dr Hector J D'Agnillo (Argentina) 4
-
Hospital
Provincial Neuquen (Argentina) 4
-
Chesterfield
& North Derbyshire Royal Hospital (UK) 4
-
Prof
Dr D Bagdasar Clinical Emergency Hospital (Romania)4
-
City
Hospital (UK) 4
-
Instituto
de Prevision Social (Paraguay) 3
-
Chelsea
and Westminster Hospital (UK) 3
-
KAT
Hospital of Athens (Greece) 3
-
José
Penna (Argentina) 3
-
Hospital
"Heroes de Malvinas" (Argentina) 2
-
Doncaster
Royal Infirmary (UK) 2
-
Narh-Bita
Hospital (Ghana) 2
-
Arrowe
Park Hospital (UK) 1
-
Hospital
Español (Argentina) 1
-
Univerzity
Karlovy Neurochirurgicka Klinika (Czech R) 1
-
Hospital
Nacional Profesor Alejandro Posadas (Argentina) 1
-
Ospedale
San Martino (Italy) 1
-
Institute
Of Neurology (UK) 1
-
Kantonsspital
Schaffhausen (Switzerland) 1
-
Harrogate
District Hospital (UK) 1
-
King
George Hospital (UK) 0
-
University
Hospital of Brooklyn (USA) 0
-
St
Helier Hospital (UK) 0
-
North
Staffordshire Royal Infirmary (UK) 0
-
MetroHealth
Medical Center (USA) 0
-
Makerere
Medical School (Uganda) 0
-
Istambul
Medical Faculty (Turkey) 0
-
Kreiskrankenhaus
Tirschenreuth (Germany) 0
-
Clinica
Los Rosales S.A. (Colombia) 0
-
Hospital
Universitario San Jorge (Colombia) 0
-
Hospital
Mútua de Terrassa (Spain) 0
-
Lister
Hospital (UK) 0