|
|
|||||
| trial in the news | |||||
| News
about the CRASH Trial is spreading fast
Following a news item in the BMJ earlier this month there has been strong interest from neurosurgeons and emergency physicians around the world. Two large South African neurosurgical units are applying for ethics committee approval with several potential new collaborators in Italy, Poland, Slovakia, and the UK. A further opportunity to extend the network of collaborators might also result from the favourable review of the CRASH protocol by The Lancet. For the past few years The Lancet has operated a system of peer review of protocols. The aim of this system is threefold: to encourage good principles in the design of clinical research, to publicise a list of "accepted" protocols, and to make a provisional commitment to publication of the main clinical endpoints of the study. The journal looks for trials that address an important question and in which the planned design and analysis are original, topical, and valid. The journal sends selected protocols for external peer review by clinical advisors and by statisticians and then lists on its web-site details of protocols that pass scrutiny. Having passed this hurdle, details about the CRASH trial will soon be posted on The Lancet web-site. |
|||||
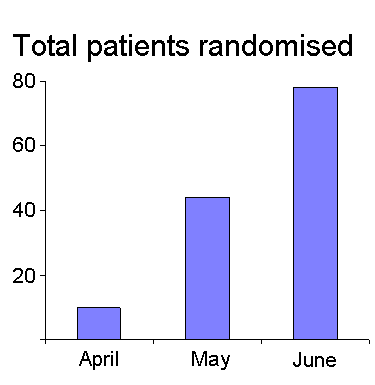
| RECRUITMENT
This has really taken off with the number of patients randomised doubling in the last month. To see the most up to date numbers, the total number of patients entered is regularly updated on our web site: Jimmy Stuart, North Manchester Hospital and Jiri Occhman in Brno, Czech Republic have both made incredible efforts, pushing the numbers well up. Scotland has now gone live with Mr. Matheson's team, in Aberdeen having randomised their first patient into the trial this month. Leeds General Infirmary is expected to start randomising soon. Italy have four centres with Ethics Committee approval and treatment packs on site. Randomisation is imminent. |
 |
||||
| EARLY
OUTCOME FORMS
Early outcome forms are being returned within the 14 day period. Could everyone when filling it out please complete: Question 4 - Outcome. Tick the box and also write in the date of death, discharge or transfer. Other than this the amount of missing data is minimal. |
|||||
| THE CRASH TRIAL: FIRST TWO MONTHS OF RECRUITMENT | |||||

David Yates – Professor of Emergency Medicine at Hope Hospital, Manchester |
First
it was concern – and not a little confusion – as we struggled to put into
practice all that we had planned in the months of preparation for the trial.
Who would be responsible for enrolment throughout the 24 hours? Would the
trial packs fit into the resus room mini pharmacy? Had we spoken to all
key staff members? (We hadn’t.) Would the CRASH forms become submerged
under all the protocols, student project questionnaires and registrar research
studies which compete for space on the emergency department work surfaces?
The answers were often uncomfortable but the determination was there and
concern is now maturing into competition as our enrolment rate increases
and into confidence as we gain experience in dealing with practical issues.
This learning curve is an inevitable feature of the early phase of a large ‘simple’ trial in an emergency setting. But, it is reassuring that the slope is steep and that quite a few centres have now reached double figures and are settling down to the business of recruitment in large numbers. I hope that readers who are cautiously beginning this process are reassured that the CRASH methodology will ensure the safety of their patients and provide a simple but elegant way of resolving the ‘steroid issue’ in traumatic brain injury once and for all. |
||||
|
CRASH Co-ordinating Centre, FREEPOST LON 14211, LONDON WC1N 1BR Tel: + 44 (0)20 7299 4684 Fax: + 44 (0)20 7299 4663 email: CRASH@lshtm.ac.uk
|
|||||