|
|
||
Keep randomising over the millennium! |
||
| Randomising
over the Millenium
Between 5pm on Thursday 30th December 1999 and 10am on Tuesday 4th January 2000, the telephone randomisation service will be out of action. During this period the systems are being tested because of 'Y2K' concerns. To randomise a patient during this period, take the next numbered treatment pack, fill in a Patient Entry form, putting the date and time of randomisation and the treatment pack number on the form, and then fax it to the CRASH Co-ordinating Centre (+44(0) 207 242 2723). We will provide new Patient Entry forms for this. They are almost identical to the existing forms but have a space at the top of the page for the date and time of randomisation. For unblinding please continue to contact the 24-hour FREEPHONE randomisation service, who will be available to manage this service. Please contact Nin if you have any questions about this – materials will be arriving shortly! |
||
| There are now well over 200 patients in the trial | ||
In
keeping with the inclusion criteria, there is a broad range of head injury
severity in the trial, with approximately equal numbers of mild, moderate
and severe head injuries. This is an important strength.
 |
Because of the
range of head injury severity in the trial it should be possible to determine
which types of patient are most likely to benefit from treatment.
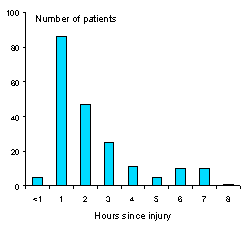
As regards timing, results from animal studies show that early administration of corticosteroid is important for maximal effect. Early administration of corticosteroid has also been shown to be important in acute spinal cord injury. That 80% of patients in CRASH were treated within three hours from injury is very encouraging. |
|
| Data monitoring
committee says well done and keep going...
All MRC trials have a Data Monitoring and Ethics Committee to look at the trial data from an ethical standpoint and to ensure that the safety, rights and well being of the trial participants is paramount. The Committee met in October to review the data from the first 166 patients recruited to the trial. It was the view of the committee that there was no reason to recommend any changes to the study procedures or plans for continued patient recruitment. The Committee congratulated collaborating investigators on an excellent start to recruitment. |
||
|
CRASH Co-ordinating Centre, FREEPOST LON 14211, LONDON WC1N 1BR Tel: + 44 (0)20 7299 4684 Fax: + 44 (0)20 7299 4663 email: CRASH@lshtm.ac.uk |
||