| Trial Protocol | Back |
Study Design.
| Summary |
|
CRASH is a large simple, placebo controlled trial of the effects of a 48-hour infusion of corticosteroids on death and on neurological disability, among adults with head injury and some impairment of consciousness. Head injured patients with impaired consciousness who are judged to be 16 years or older are eligible if the responsible doctor is for any reason substantially uncertain whether or not to use corticosteroids. Numbered drug or placebo packs will be available in each participating emergency department. Randomisation involves calling a 24-hour freephone service. The call should last only a minute or two, and at the end of it the service will specify to the caller which numbered treatment pack to use. The drug or placebo in the pack is made up in saline and, following a one-hour loading dose, is infused over 48 hours (or as close to 48 hours as possible). No extra tests are required, but a short form is to be completed two weeks later (or after prior death or discharge). Long term recovery will be assessed at six months either by a simple questionnaire, sent directly to each trial participant from the CRASH Co-ordinating Centre, or by telephone interview. This does not involve any additional work for collaborating hospitals.
|
| Number of Patients Needed |
Two main factors
determine the number of patients needed in a trial. These are the estimated
event rate and the size of the treatment effect. |
|
Estimated
event rate: In a recent multi-centre randomised
trial in head injury using inclusion criteria similar to those in the
CRASH trial, the overall risk of death among controls was 15%, with
the risk of unfavourable outcome (dead, unfit for work or needing rehabilitation)
being 43%.[Ref 12]
This trial is one of the most recent randomised trials of corticosteroids
in head injury and it would be reasonable to expect a similar risk of
unfavourable outcome in the CRASH trial. |
Size
of treatment effect that should be detectable: Because
even a 2% survival advantage for an intervention as simple and widely
practicable as corticosteroids would represent a worthwhile benefit, the
current trial has been planned to be able to detect a benefit of this
size. |
Numbers
needed: If the real mortality difference is 15% vs 13%
then there is about a 65% chance that a trial involving 10,000 patients
will achieve 2P<0.01, and a 95% chance that a trial involving 20,000
patients will do so. These calculations assess how well the trial is protected
against an unfavourable play of chance. If however, as might well be the
case, the actual results are not much distorted by the play of chance
and involve 15% vs 13% mortality then a trial of 10,000 would yield 2P=0.004,
and a trial of 20,000 would yield 2P=0.00004 (which is extreme enough
to allow some exploratory sub-analyses of which types of patient seem
most likely to benefit). |
| Eligibility |
|
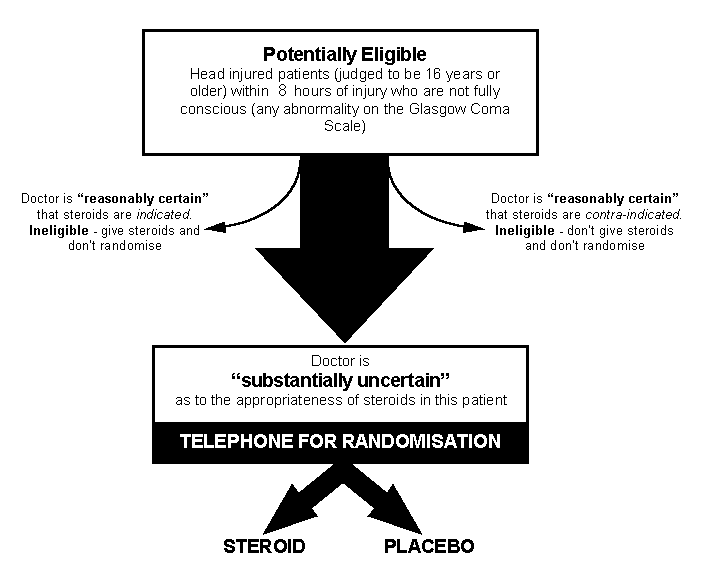
Head injured patients (judged
to be 16 years or older) within 8 hours of injury who are not fully
conscious (any abnormality on the Glasgow Coma Scale), except those
for whom corticosteroids are thought to be clearly indicated or contra-indicated. |
All head injured
patients who - in the absence of sedation - are observed whilst in hospital
to have GCS of 14 or less, and are within 8 hours of the injury, are eligible
for trial entry if they appear to be at least 16 years old. Although entry
is allowed up to 8 hours from injury, the earlier that patients can be
treated the better. |
|
There
are no other pre-specified exclusion criteria, as the fundamental eligibility
criterion is the responsible doctor's "uncertainty" whether or not to
use corticosteroids in a particular adult with head injury.[Ref
13] Patients for whom there is considered by the responsible
doctor to be a clear indication for corticosteroids (such as, perhaps,
those who also have an acute spinal cord injury) should not be
randomised. Likewise, any for whom there is considered to be a clear
contraindication to corticosteroids should not be randomised.
But, all those for whom the responsible doctor is substantially uncertain
as to whether or not to give corticosteroids are eligible for randomisation,
and as many such patients as possible should be considered for the trial.
|
Heterogeneity of
the types of patients entering such a trial is a scientific strength,
not a weakness. If a wide range of patients are randomised then it may
be possible for a really big trial such as this one to help determine
which (if any) particular types of patient are most likely to benefit
from treatment. |
 |
Special eligibility
considerations: None. Routine exclusion of patients with gastrointestinal
complaints or pregnancy is unnecessary, unless the responsible doctor
considers these to be a definite contraindication. |
Notes:- |
| Consent |
|
Patients with head
injury and impaired consciousness may be unable to give properly informed
consent, and in this emergency situation it may not be medically appropriate
to delay the start of treatment until proxy consent can be obtained.
Hence, the doctor in charge should take responsibility for entering
such patients, just as they would take
|
|
Patients eligible for inclusion should be randomised, and the study treatment started, as soon as possible. Randomisation is done by telephoning a 24-hour toll-free service and takes only about two minutes. The questions that will be asked by the telephone operator prior to allocation of the treatment packs are shown in [Appendix 2] . The study computer will then randomly assign a treatment pack number that will identify one of the CRASH treatment packs stored in the emergency department. Once a patient has been randomised, we will definitely wish to learn the outcome in hospital, even if the trial treatment gets interrupted or is not actually given.
|
| Study Treatment |
| Each CRASH treatment pack contains: |
|
| Treatment |
|
Dose (MP or placebo) |
| Loading |
|
2g over 1 hour |
| Day 1 |
|
0.4 g/hour for ~24 hours |
| Day 2 |
|
0.4 g/hour for ~24 hours |
Loading |
2g MP (or matching placebo) over 1 hour in 100mL infusion: |
1. Add 20mL water
for injection to one 2g vial and mix well |
Daily Maintenance |
| 0.4g/hour for about 24 hours in 20mL/hour infusion (MP or matching placebo): |
1. Remove 100mL from
a 500mL bag of 0.9% NaCl (to make room for the steroid) |
N.B. As children
under 16 are excluded, a simple fixed-dose treatment can be used.
The dosing regimen is that used in the NASCIS-2 and NASCIS-3 trials of
MP in acute spinal cord injury. |
| Unexpected Adverse Events |
|
Anaphylactic reactions to intravenous corticosteroids are extremely rare, but should be treated in whatever way the responsible doctor prefers (one possibility being intra-muscular adrenaline 0.5mg, i.e. 0.5 mL of 1 in 1,000 (1mg/mL) solution).[Ref 14] It would be expected that 24-hour anaesthetic cover would be available in all hospitals participating in CRASH. If a serious and unexpected adverse drug reaction occurs and is suspected to be related to the study medicine, this should be logged by calling the 24-hour randomisation service, who will inform the CRASH Co-ordinating Centre in London. |
|
In general, gastro-intestinal bleeds and infections do not need to be reported in this way because some increase in their incidence might be expected with steroids. Likewise, the various medical events that are to be expected in head injured patients do not need to be reported by telephone. All such events are, however, routinely monitored among all patients on the Early Outcome Form [Appendix 3].. . |
| Unblinding |
|
In general there should be no need to unblind the allocated treatment. If some contra-indication to corticosteroids develops after randomisation (e.g. severe gastro-intestinal bleeding), the trial treatment should simply be stopped. Unblinding was never found to be necessary in the NASCIS trial of MP in spinal cord injury,[Ref 4] and should be done only in those rare cases when the doctor believes that clinical management depends importantly upon knowledge of whether the patient received corticosteroid or placebo (e.g. suspected anaphylaxis). In those few cases when urgent unblinding is considered necessary, the randomisation service should be telephoned, giving the name of the doctor authorising unblinding and the CRASH treatment pack number (if available), and the caller will then be told whether the patient received corticosteroid or placebo.
|
The primary outcome
measures are: |
|
In-hospital deaths,
complications and short-term recovery are to be recorded on the Early
Outcome Form which can be completed entirely from the hospital notes -
no extra tests are needed. |
|
Long term recovery will be assessed at six months using the Glasgow Outcome Scale (GOS), which assesses disability and handicap in major areas of life. The GOS will be administered by a postal questionnaire [Appendix 4], completed by the patient or a carer, or by telephone interview. (This does not involve any additional work for the collaborating hospitals.)
|
| Analysis |
Comparisons will be made of the primary outcome measures, comparing all those allocated methylprednisolone versus all those allocated placebo, on an 'intention to treat' basis. Analyses will be stratified on time from injury to the initiation of treatment, and on severity of head injury as assessed by the Glasgow Coma Scale. Comparisons will also be made of the risks of infection and gastrointestinal bleeding. |